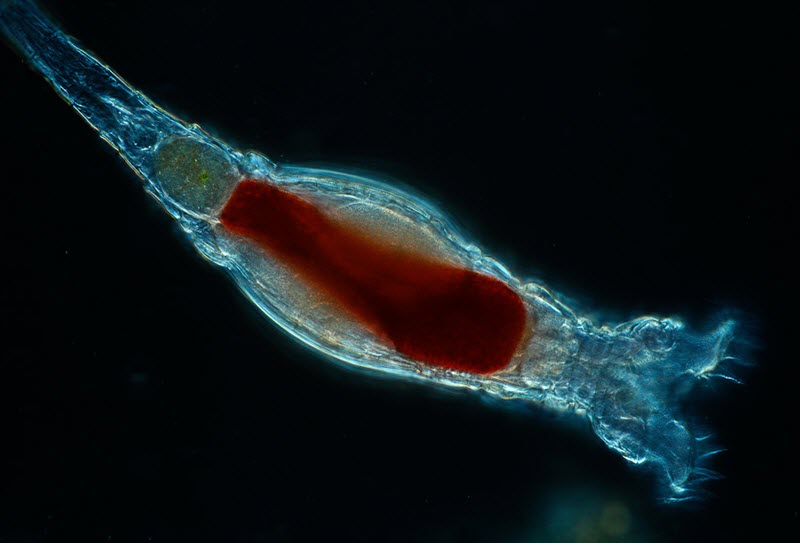
Scientists with the Marine Biological Laboratory have discovered a new form of genetic modification carried out by tiny animals called bdelloid rotifiers. This form of genetic modification has previously only been observed in bacteria, and researchers believe that a bdelloid rotifier incorporated it from a bacterium some 60 million years ago.
About bdelloid rotifiers
Bdelloid rotifiers, commonly called bdelloid wheel animals, are microscopic animals that live in freshwater environments world wide and rely exclusively on parthenogenetic reproduction. Because of their unusual reproductive style, which spans back to over 25 million years ago, they are referred to as “the ancient asexuals”. Bdelloid rotifiers are typically 150-700 µm in length. Most are too small to notice with the naked human eye, but it doesn´t take a very powerful magnifying glass to observe them as tiny white dots under a bright light. Bdelloid rotifiers can enter into anhydrobiosis (a type of dormancy) at any life stage to survive in harsh conditions, and in June 2021 biologists reported that they had restored bdelloids that had remained frozen in the Siberian permafrost for 24,000 years.
A third epigenetic mark; acquired from bacteria
My DNA is not just the blueprint for my body; it is also a living document where adjustments to the design can be made by epigenetic marks. When researchers catalogue these marks and what impact they have, they earn a better understanding of genetics.
In eukaryotes – organisms whose cells have a nucleus enclosed within a nuclear envelope – researchers know of the principal epigenetic marks. (The domain Eukaryota is one of the three domains of life; bacteria and archaea (the prokaryotes) are the other two domains.)
Now, researchers at the University of Chicago-affiliated Marine Biological Laboratory have discovered a third epigenetic mark in bdelloid rotifiers. This is surprising, as bdelloid rotifiers are animals and belong to the domain Eukaryota, while this third type of epigenetic mark was formerly only known to exist in bacteria.
The surprising discovery was reported in an article published in Nature Communications on February 28, 2022.
The presence of the third type of epigenetic mark is believed to have been absorbed from bacteria that were in close contact with bdelloid rotifiers.
“We discovered back in 2008 that bdelloid rotifers are very good at capturing foreign genes,” said senior author Irina Arkhipova, senior scientist in the Marine Biological Laboratory’s Josephine Bay Paul Center. “What we’ve found here is that rotifers, about 60 million years ago, accidentally captured a bacterial gene that allowed them to introduce a new epigenetic mark that was not there before.”
This is the scientific world´s first – and so far only – report of a horizontally transferred gene being able to reshape the gene regulatory system in an eukaryote.
“This is very unusual and has not been previously reported,” Arkhipova said. “Horizontally transferred genes are thought to preferentially be operational genes, not regulatory genes. It is hard to imagine how a single, horizontally transferred gene would form a new regulatory system, because the existing regulatory systems are already very complicated.”
“It’s almost unbelievable,” said co-first author Irina Yushenova, a research scientist in Arkhipova’s lab.
What are epigenetic marks?
Epigenetic marks are modifications to DNA bases that do not change the underlying genetic code. In a sense, the epigenetic mark is “written on top” of the genetic code.
Just like the genetic code is inherited, epigenetic marks can be inherited.
What can epigenetic marks do?
- Regulate gene expression by turning genes on or off. Research indicate that this is extra common during early development, but also when the body is under stress.
- Supress transposons (“jumping genes”) that may threaten the integrity of the genome.
A complex process
As stated above, having a single horizontally transferred gene actually form a new regulatory system within the receiving organism is hard to imagine, especially considering how complex the existing regulatory systems are in eukaryote organisms. Still, that seems to be exactly what happened in a bdelloid rotifier some 60 million years ago.
Co-first author Irina Yushenova explained how this process would have occurred: “Just try to picture, somewhere back in time, a piece of bacterial DNA happened to be fused to a piece of eukaryotic DNA. Both of them became joined in the rotifer’s genome and they formed a functional enzyme. That’s not so easy to do, even in the lab, and it happened naturally. And then this composite enzyme created this amazing regulatory system, and bdelloid rotifers were able to start using it to control all these jumping transposons. It’s like magic.”
Transposons are genes that move around from one place to another inside your genome, and each such movement can change the genetic code. Sometimes the change turns out to be beneficial for the organism, sometimes negative, and sometimes have no or minimal impact. Since there can be negative outcomes, keeping the transposons under control is important.
And for bdelloid rotifers, the issue is even more pressing, as they reproduce asexually.
“Bdelloid rotifers, especially, have to keep their transposons in check because they primarily reproduce asexually,” Arkhipova said. “Asexual lineages have fewer means for suppressing proliferation of deleterious transposons, so adding an extra layer of protection could prevent a mutational meltdown. Indeed, transposon content is much lower in bdelloids than it is in sexual eukaryotes that don’t have this extra epigenetic layer in their genome defense system.”
It thus seems like the transferred gene from the bacterium proved to be highly beneficial for the bdelloid rotifier, and gave it a strong evolutionary advantage by giving it an extra mechanism for keeping transposons under control.
“You don’t want transposons jumping around in your genome,” said the study’s first author, Fernando Rodriguez, who is a research scientist in Arkhipova’s lab. “They will mess things up, so you want to keep them in check. And the epigenetic system to accomplish that is different in different animals. In this case, a horizontal gene transfer from bacteria into bdelloid rotifers created a new epigenetic system in animals that hasn’t been described before.”
Reference
“Bacterial N4-methylcytosine as an epigenetic mark in eukaryotic DNA” by Fernando Rodriguez, Irina A. Yushenova, Daniel DiCorpo and Irina R. Arkhipova, 28 February 2022, Nature Communications.
DOI: 10.1038/s41467-022-28471-w
https://www.nature.com/articles/s41467-022-28471-w
(Länken innehåller diagram etc om du vill ha någon bild till artikeln.)
Founding for the project came from the National Institutes of Health.
The Marine Biological Laboratory is a private, non-profit institutes and an affiliate of the University of Chicago.