Understanding DNA Methylation
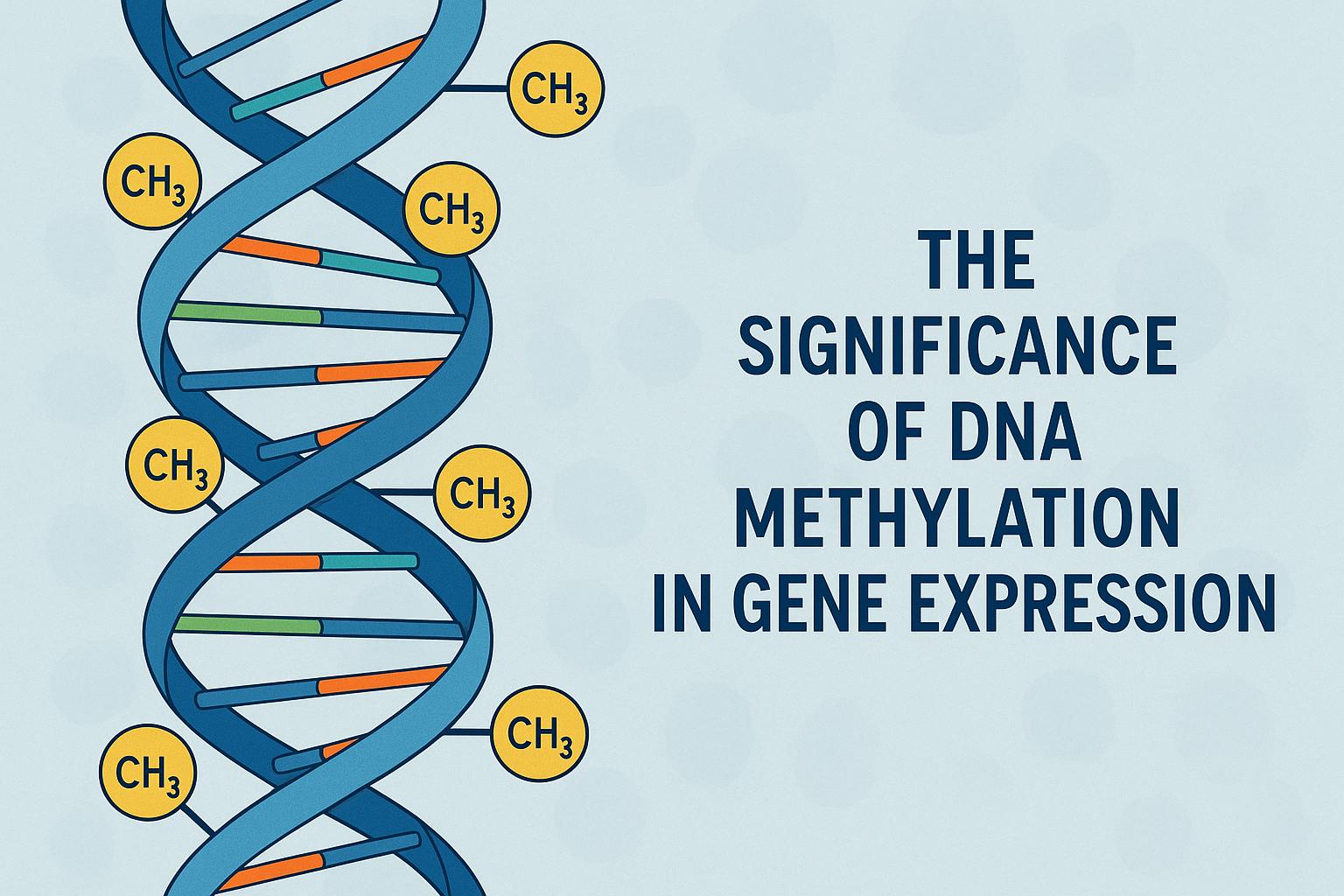
DNA methylation plays a crucial role in the regulation of gene expression. This biochemical process involves the addition of a methyl group to the cytosine base in DNA, often influencing how genes are expressed in cells. Understanding this mechanism is essential for deciphering the complexities of genetic regulation and its implications for health and disease.
The Role of DNA Methylation in Gene Regulation
DNA methylation is a key epigenetic modification that affects gene expression without altering the DNA sequence. This process typically occurs at CpG sites, where a cytosine nucleotide is followed by a guanine nucleotide. Methylation at these sites usually leads to gene silencing, as it can inhibit the binding of transcriptional machinery to DNA, thereby reducing gene expression.
Impact on Development and Differentiation
During development, DNA methylation patterns are established and maintained to ensure that specific genes are expressed in a cell-type and tissue-specific manner. This is critical for cell differentiation, where cells become specialized in structure and function. Abnormal methylation patterns can lead to developmental disorders and have been associated with various diseases, including cancer.
The Connection with Cancer
Aberrant DNA methylation is a hallmark of cancer, with regions of hypermethylation often observed in tumor suppressor genes, leading to their inactivation. Conversely, global hypomethylation can activate oncogenes and contribute to genomic instability. Understanding these patterns helps in developing potential therapeutic approaches targeting epigenetic modifications.
Mechanisms and Biochemical Pathways
DNA methylation primarily occurs through the transfer of a methyl group from S-adenosylmethionine (SAM) to the cytosine ring of DNA, a process catalyzed by DNA methyltransferases (DNMTs). DNMT1 maintains methylation patterns during DNA replication, while DNMT3A and DNMT3B are responsible for de novo methylation, establishing new patterns during early development. Dysregulation in these enzymes can lead to incorrect methylation, with widespread effects on gene expression and cellular functions.
Environmental and Lifestyle Influences on Methylation
DNA methylation is not solely determined by genetic factors. Environmental factors, including diet, exposure to toxins, and lifestyle choices, can influence methylation patterns. For instance, folate and other B-vitamins are critical for synthesizing SAM, the primary methyl donor, linking nutrition directly to methylation status. Additionally, exposure to cigarette smoke, heavy metals, and other pollutants can lead to aberrant methylation, which may contribute to the onset of disease.
Inheritance and Transgenerational Effects
Methylation patterns can be inherited through generations, influencing gene expression in descendants. Studies have shown that changes in parental methylation due to environmental factors can be passed down, affecting offspring health. This transgenerational inheritance suggests a mechanism by which environmental impacts can have long-term consequences beyond immediate exposure, highlighting the complex interplay between genetics and the environment.
Emerging Technologies in Methylation Research
Recent advances in genomic technologies have revolutionized the study of DNA methylation. Techniques such as bisulfite sequencing allow researchers to map methylation at single-base resolution across the entire genome. These approaches have elucidated the plasticity of the epigenome, showing how methylation can be dynamically altered in response to various stimuli. The development of CRISPR-based systems to edit methylation at specific loci also offers exciting tools for probing the functional consequences of methylation changes.
Therapeutic Potential and Challenges
The potential for epigenetic therapies targeting DNA methylation is a rapidly developing field. Drugs that inhibit DNMTs, known as DNMT inhibitors, are currently in use for certain types of cancer, aiming to reactivate silenced tumor suppressor genes. However, challenges persist, including the precise delivery of these drugs and the need to avoid unintended effects on normal gene regulation. Balancing the therapeutic benefits of altering methylation with potential risks remains a significant challenge.
Clinical Applications and Personalized Medicine
In clinical settings, methylation patterns serve as biomarkers for diseases, helping in the diagnosis and prognosis of conditions such as cancer. The methylation status of specific genes can indicate disease risk or reveal insights into the disease subtype and likely progression. Personalized medicine approaches are increasingly incorporating methylation profiling to tailor therapies based on an individual’s unique epigenetic landscape. This personalization holds promise for improving treatment outcomes and minimizing adverse effects.
Ethical Considerations in Epigenetics
As research in DNA methylation and its applications advances, ethical considerations come to the forefront. The ability to alter epigenetic markings such as methylation could have profound implications, raising questions about consent, privacy, and potential misuse. The concept of editing epigenetic memory, particularly when considering heritable changes, requires careful ethical scrutiny. As the field progresses, balancing scientific opportunity with ethical responsibility is crucial for the responsible development of epigenetic technologies.
In summary, DNA methylation is a significant epigenetic mechanism regulating gene expression. It holds profound implications for understanding development, disease etiology, and the development of novel therapeutic approaches. As research continues to unravel the complexities of this process, the landscape of medical treatment, diagnosis, and ethical considerations will likely continue to evolve, offering new insights and challenges in the realm of genetic and epigenetic research.